Question 1
The volume of seawater on Earth is about 330,000,000 m3 If seawater is 3.5% sodium chloride by mass and has a density of 1.03 g/mL what is the approximate mass of sodium chloride, in tons, dissolved in the seawater on Earth (1 ton = 2000 lb)
Question 2
The diameter of metal wire is often referred to by its American wire-gauge number. A 16-gauge wire has a diameter of 0.05082 in. What length of wire, in meters, is found in a 1.00 lb spool of 16-gauge copper wire? The density of copper is 8.92 g/cm3.
Question 3
A typical rate of
deposit of dust (“dustfall”) from unpolluted air was
reported as 10 tons per square mile
per month. (a) Express this dustfall in milligrams per square meter per hour. (b) If the dust has an average density of 2 g/cm3,
how long would it take
to accumulate a layer of dust 1 mm thick?
Question 4
A Fahrenheit and a Celsius thermometer are immersed in the same medium. At what Celsius temperature will the numerical reading on the Fahrenheit thermometer be (a) 49° less than that on the Celsius thermometer; (b) twice that on the Celsius thermometer; (c) one-eighth that on the Celsius thermometer; (d) 300° more than that on the Celsius thermometer?
Question 5
A pycnometer
(see Exercise 78) weighs 25.60 g empty and 35.55 g when filled with water at 20
°C. The density of water at 20 °C is When 10.20 g of lead is placed in the
pycnometer and the pycnometer is again filled with water at 20 °C, the total
mass is 44.83 g. What is the density of the lead in grams per cubic centimeter?
Question 6
The Greater Vancouver Regional District (GVRD) chlorinates the water supply of the region at the rate of 1 ppm, that is, 1 kilogram of chlorine per million kilograms of water. The chlorine is introduced in the form of sodium hypochlorite, which is 47.62% chlorine. The population of the GVRD is 1.8 million persons. If each person uses 750 L of water per day, how many kilograms of sodium hypochlorite must be added to the water supply each week to produce the required chlorine level of 1 ppm
Question 7
A piece of
high-density Styrofoam measuring 24.0 cm by
36.0 cm by 5.0 cm floats when placed in a tub of water. When a 1.5 kg book is placed on
top of the Styrofoam, the
Styrofoam partially sinks, as illustrated in the diagram below. Assuming that the
density of water is 1.00
g/mL, what is the density of Styrofoam?
Question 8
The total volume of ice in the Antarctic is about 3.01 107 km3. If all the ice in the Antarctic were to melt completely, estimate the rise, h, in sea level that would result from the additional liquid water entering the oceans. The densities of ice and fresh water are 0.92 g/cm3 and 1.0 g/cm3, respectively. Assume that the oceans of the world cover an area, A, of about 3.62 108 km2 and that the increase in volume of the oceans can be calculated as A x h.
Question 9
An empty 3.00 L bottle weighs 1.70 kg. Filled with a certain wine, it weighs 4.72 kg. The wine contains 11.5% ethyl alcohol by mass. How many grams of ethyl alcohol are there in 250.0 mL of this wine?
Question 10
The filament in an incandescent light bulb is made from tungsten metal (d 19.3 g/cm3) that has been drawn into a very thin wire. The diameter of the wire is difficult to measure directly, so it is sometimes estimated by measuring the mass of a fixed length of wire. If a 0.200 m length of tungsten wire weighs 42.9 mg, then what is the diameter of the wire? Express your answer in millimeters.
Question 11
Blood alcohol content (BAC) is sometimes reported in weight-volume percent and, when it is, a BAC of 0.10% corresponds to 0.10 g of ethyl alcohol per 100 mL of blood. In many jurisdictions, a person is considered legally intoxicated if his or her BAC is 0.10%. Suppose that a 68 kg person has a total blood volume of 5.4 L and breaks down ethyl alcohol at a rate of 10.0 grams per hour.* How many 145 mL glasses of wine, consumed over three hours, will produce a BAC of 0.10% in this 68 kg person? Assume the wine has a density of 1.01 g/mL and is 11.5% ethyl alcohol by mass. (*The rate at which ethyl alcohol is broken down varies dramatically from person to person. The value given here for the rate is a realistic, but not necessarily accurate, value.)
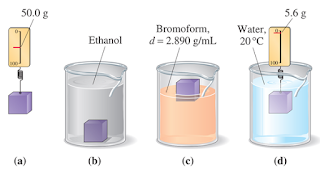
Question 12
The accompanying sketches suggest four observations made on a small block of plastic material. Tell what conclusions can be drawn from each sketch, and conclude by giving your best estimate of thedensity of the plastic.
- 5.5 x 1016 tons
- 38.8 m
- a. 74 b. 4.1 x 105 h
- a. -101.25 ºC b. 160 ºC c. -19.1 ºC d. 335 ºC
- 11 g/mL
- 1.98 x 104 kg sodium hypochlorite
- 70.25 g/cm3
- 76.4 m
- 28.9 g ethanol
- 0.119 mm
- 2.1 glasses of wine
- The information provided in sketches (a) and (d) allows calculation of the density. Densityof the plastic material = 1.12 g/cm3

Post a Comment