There are primarily three applications for Lewis structures. They are useful for familiarising you with the placement of electrons around atoms, visualising molecular geometry, and remembering the location of lone pairs. As you'll see in Org 1, the geometry of molecules and the location of lone pairs have a significant impact on reactivity; therefore, it would be beneficial to review these concepts using Lewis structures.
What do Lewis structures entail?
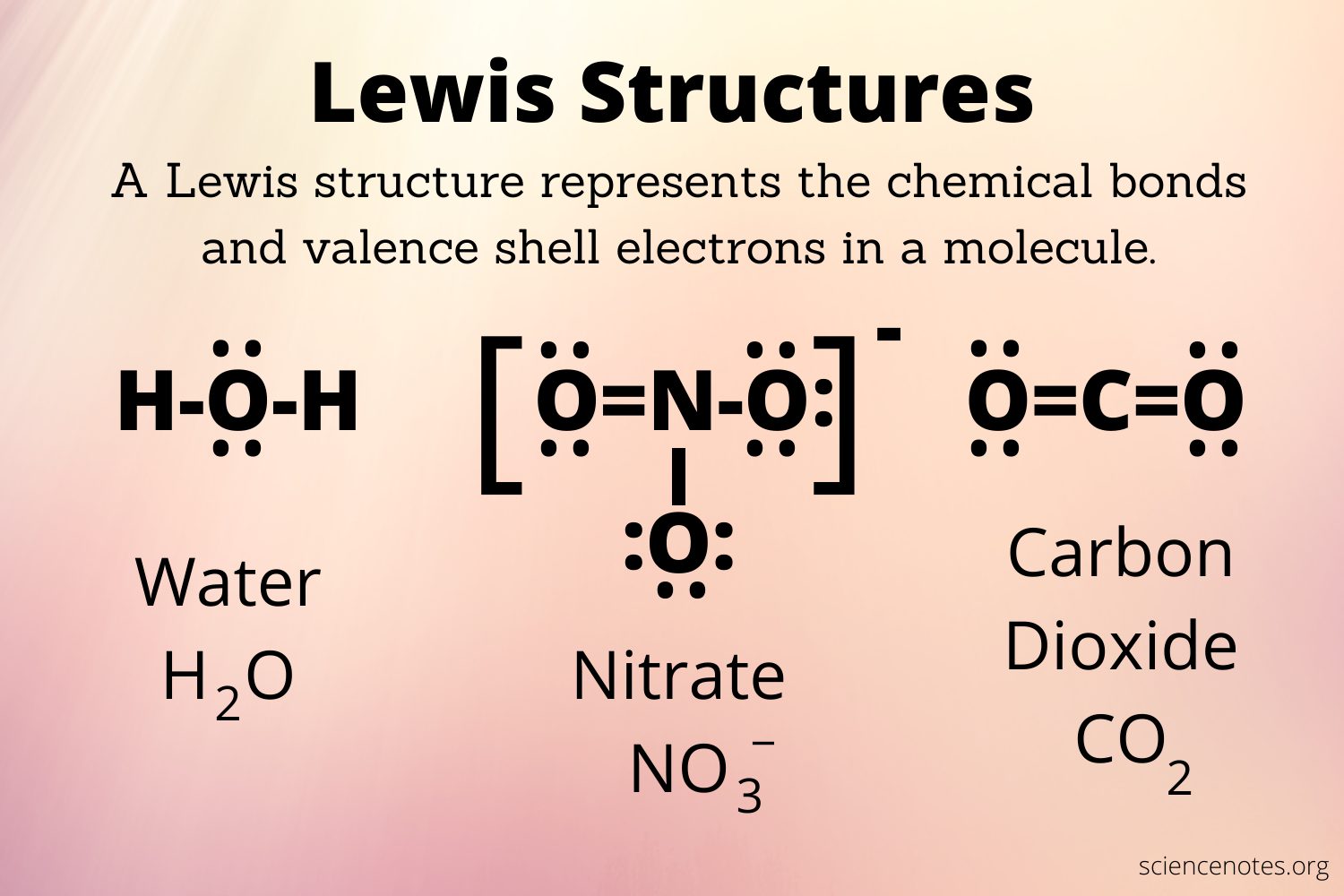
They are a method for depicting molecules that illustrates the location of electrons between atoms. For example, the lewis dot structures of beryllium dichloride, borane, methane, ammonia, water, and hydrofluoric acid are provided below.
The advantage of the full Lewis is that it enables you to determine the location of all the electrons and whether or not each atom adheres to the octet rule. Molecules can contain both bonding electrons, which are shared between atoms, and non-bonding electrons, also known as lone pairs.
The full Lewis is comparable to bicycle training wheels. It's helpful when you're just starting out and feeling unsure about atoms, electrons, and molecules, and you want to determine for yourself that the octet rule is indeed a widespread phenomenon that (most) molecules adhere to [barring electron-deficient beryllium and boron].
However, you will quickly realise that drawing a complete Lewis structure is a bit tedious. As shown below, once you are familiar with the fundamentals of drawing molecules, it becomes much easier to simply draw a line where the bond is. This also has the benefit of making it easier to illustrate geometry using line bonds, as there is less clutter.
We now reach our second point. Electron pairs repel; this holds true for both bonded and lone electron pairs. Therefore, molecules will adopt a geometry that maximises their distance apart. This explains why methane is tetrahedral (internal angles of 109°) rather than square planar (internal angles of 90°) and why water is curved rather than linear. This phenomenon is known as VSEPR (valence shell electron pair repulsion).
Using a full Lewis diagram to depict molecular geometry results in a drawing that is extremely cluttered. Therefore, the full Lewis is replaced by the half-Lewis, the lines are rearranged, and only the electron pairs are retained.
Now, even a second level of laziness exists. It is less laborious to omit drawing electron pairs altogether. This is by far the most common method for drawing molecules. Let's examine some examples.
Lewis structures where lone pairs are implied should not contain hidden lewis pairs, such as formaldehyde and water. despite the fact that lone pairs were not drawn, they still exist.
Even though the lone pairs are not drawn in, you should be aware that they remain. Consider them chemical stick figures. For example, xkcd typically does not draw faces, feet, or hands, but that does not imply that the characters he draws are faceless amputees. Simply put, it is faster to draw stick figures.This is significant because, in organic chemistry, lone pairs rarely sit idle. As you will see in the following section, lone pairs are nucleophiles and participate in a variety of chemical reactions. Therefore, it is essential to recognise their presence, even if they are not interested.
Post a Comment