What Orbitals Play a Role in Methane's (CH4) Tetrahedral C-H Bond Arrangement Around Carbon?
Hybrid Orbitals: A Justification For Carbon-Based Bonding
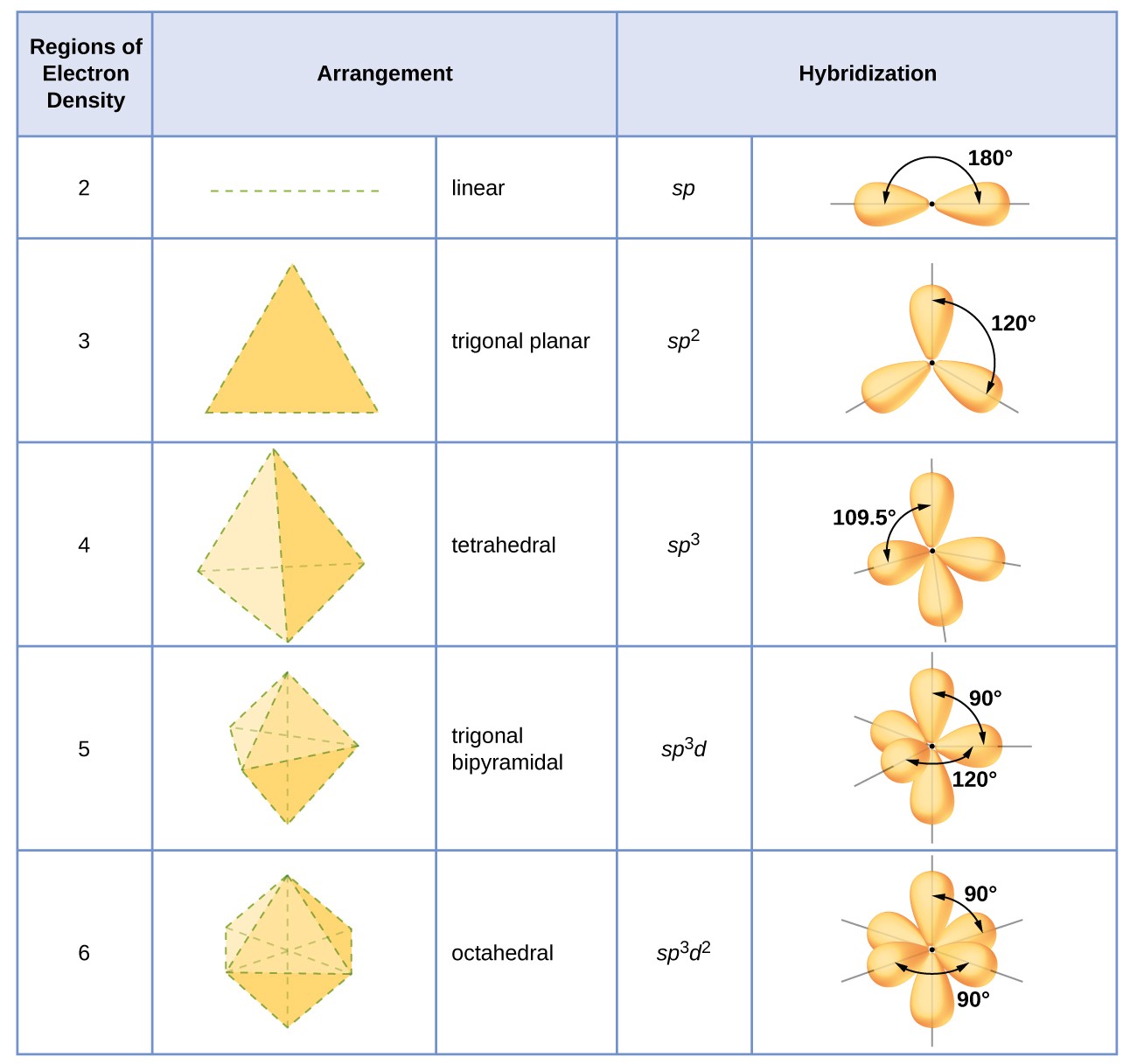
A popular analogy The First-Row Elements With Atoms Bonded To Four Atoms Are Described Accurately By Hybrid Soda Sp3 Hybridization, as Are Situations Where [Atoms + Lone Pairs] Equals 4 spHybridization step 2 Summary of Hybridization - Hybrid Orbitals
1. What Orbitals Are Involved In The Tetrahedral Arrangement Of Methane (CH4)?
How do we know that methane is tetrahedral was a question raised in the previous post on the structure of methane (See Article: How Do We Know That Methane Is Tetrahedral).
We might have erroneously assumed, based on the orbitals of carbon (2s and 2p), that three of the C-H bonds would line up along the x, y, and z axes, respectively, and the other one would be at an arbitrary angle (like about 135°).
But then, as is so common in science, some annoying experimental facts disproved our lovely intuitive hypothesis:
Methane lacks a detectable dipole moment (our "reasonable" structure, below left, would be expected to have one), whereas diamond has a tetrahedral crystal structure with identical bond lengths and 109.5 degree angles between the carbon atoms. If we were dealing with bonds between "pure" 2s and 2p orbitals, this isn't what we'd anticipate!
The geometry makes sense looking back. In fact, 109.5 degrees is the orientation that minimises the interactions between the four bonding pairs' repellent forces by maximising the distance between them. In other words, "opposite charges attract, like charges repel" is directly related to the geometry.
However, how can we explain the orbitals that were used to calculate the bond angle?
This is genuinely puzzling. Since there is only one pure 2s orbital, they cannot be pure 2s orbitals. Additionally, they cannot be pure p orbitals because of the 90° alignment of the p orbitals.
What kind of orbitals are these, exactly?
2. Hybrid Orbitals: An Explanation For Bonding At Carbon
The Nature of the Chemical Bond (1931) [pdf], a classic treatise by Linus Pauling that largely contributed to his 1954 Nobel Prize in Chemistry, posed the same question.
Pauling offered the following solution to this conundrum, which we still use today:
In methane, none of the bonding orbitals are exclusively s or exclusively p. They are actually hybrid orbitals with a combination of partial s and partial p characters.
The four hybrid sp3 orbitals, which are arranged tetrahedrally around the central carbon atom, are produced when the three 2p orbitals and the single 2s orbital hybridise (i.e., mix).
The s-character of the four hybrid orbitals is 25% and the p-character is 75%.
Each of these sp3 hybrid orbitals overlaps with a hydrogen 1s orbital in the case of methane to form the C-H bonds.
I'll be open here. This explanation is disliked by a lot of students.
It is undoubtedly perplexing at first. Why?
When we first learn about orbitals, I believe we naturally picture them as containers, kind of like atomic Tupperware for holding electrons, which also happen to come in a variety of adorable shapes.
The behaviour of electrons can then be compared to that of the "fruits" that jingle inside these containers (strict limit: 2 per container!). When we learn that they can move between containers within an atom or even leave the atom entirely, it doesn't come as a huge surprise to our intuition.
Our cute little sphere and dumbbell-shaped Tupperware containers have changed their shape and combined their properties, which is surprising when we open the fridge and discover them. This goes against what we would expect containers to do!
folks, welcome to the quantum world! If you're not mystified by this... It absolutely should!
Going forward, I don't expect you to fully comprehend hybridization or even "believe in" it on a theoretical or deep mathematical level. That is not required for our objectives. Pauling was a fantastic instructor. If you'd like, you can always read the original Pauling paper here.
We will be able to rationalise a lot of molecular structure, geometry, and behaviour using this hybridization model, so I only ask that you try to suspend your disbelief moving forward.
3. A Pop Analogy: Hybrid Soda
Here is what I believe to be a helpful little analogy that might help to make the point before we continue (thanks, Steven).
Consider having four bottles of soda: one Sprite (S) and three Pepsi (P) bottles.
Now picture emptying them all out, combining them, and then refilling each bottle with the resulting mixture.
According to Pop's Law of Conservation, you still have enough liquid for four bottles. Now, however, the pop is a mixture of Sprite and Pepsi rather than being pure Sprite or pure Pepsi.
Each bottle now specifically contains 25% Sprite character and 75% Pepsi character.
If you like, we can refer to this as "hybrid" pop or sp3.
Similar things happened to our 2s and 2p orbitals, in a way. We now have four orbitals with a 25% s character and a 75% p character by combining the three 2s and two 2p orbitals. (However, it's important to note that not just the contents of the bottles are evolving; their shapes also).
The last step is to position these four orbitals at the corners of a tetrahedron, allowing for the greatest possible separation between the four electron pairs (like charges repel!).
How does this justification benefit us?
It explains why methane has four identical H-C-H bond angles (109.5°), which form its tetrahedral molecular geometry.
It explains why methane has four identical C-H bond lengths and bond strengths.
The tetrahedral configuration of the electron pairs enables all partial charges to cancel (i.e., the vectors sum to zero), which explains why methane lacks a dipole moment.
Even though we won't get into it now, the model even helps to explain why some reactions take place [for example, the backside attack in the SN2 reaction occurs into the empty "antibonding" orbital 180 degrees from the bonding C-H orbital, if you've been reading ahead].
4. sp3 Hybridization Accurately Describes The Arrangement Of Atoms In (First-Row) Elements Bonded To Four Atoms
This is true for other gases as well. Any situation in which a (first-row) element is bonded to four atoms falls under this category. The most notable example we will examine is undoubtedly tetrahedral carbon, but it also holds true for tetrahedral nitrogen (such as NH4(+)) and even tetrahedral boron (such as BF4(-)).
5. As Well As Situations Where The Number of [Atoms + Lone Pairs] Equals 4
Even when one or more electron pairs are unbonded lone pairs, the orbital arrangement remains tetrahedral. (If you've heard of VSEPR theory, which is likely the case by the time you start organic chemistry, this shouldn't come as a surprise.)
For instance, the central atom of the hydronium ion (H3O+), the "methyl anion" (CH3 -), and ammonia (NH3) all have four pairs of electrons: three pairs of bonded electrons and one pair of unbonded electrons. As we've seen, tetrahedral geometry is the best configuration for placing four pairs of electrons, making the hybridization of the primary atom sp3. This results in a "piano stool" arrangement of atoms surrounding the central atom in the molecule, which we refer to as "trigonal pyramidal" geometry.
The central atom has trigonal planar molecular geometry and tetrahedral orbital geometry (sp3), to put it another way.
The bond angles in these are compressed from the ideal angle of 109.5°, which is an interesting fact. For instance, the H-N-H bond angles in ammonia are 107 degrees. We explain this by saying that a non-bonded lone pair is more repulsive than a "normal" bonding pair because it is nearer the atom and has a stronger effect.
When H2O (water), which has two lone pairs, is present, the deviation from the "ideal" bond angles is even greater. Although the resulting molecule's shape (or "molecular geometry") is "bent," the hybridization is still sp3, and the orbital geometry is still tetrahedral.
The fact that Pauling's hybridization model accurately takes into account the water's dipole moment is in fact one of its notable accomplishments. The dipoles would cancel each other out in a perfect "linear" water, as many of us may have naively believed before learning any chemistry.
The amide anion NH2 is another example of "bent" geometry because it has two lone pairs on nitrogen.
We can quickly summarise what we've learned about sp3 hybridization using the following table:
6. sp2 Hybridization
Let's revisit the pop-bottle comparison. Let's say we use two bottles of Pepsi instead of three to mix with our Sprite.
What is left over after that?
We are left with one unhybridized bottle of pop and three hybrid bottles of soda.
To illustrate, if we combine the 2s orbital with two 2p orbitals, we get three sp2 hybrid orbitals and one unhybridized p orbital that is left over.
The bond angle that maximises the distance between these three sp2 orbitals when they are all filled with electron pairs is 120°.
As a result, the sp2 orbitals are arranged in a "trigonal planar" pattern, with the unhybridized p orbital positioned perpendicular to the plane. It somewhat resembles the Mercedes Benz logo.
The chemical compound borane, BH3, which has three pairs of bonding electrons arranged at a 120° angle to one another, is a classic example of this trigonal planar geometry. Additionally, carbocations, such as the methyl cation, CH3+, exhibit the trigonal planar geometry.
Where is the unhybridized p orbital, you might be wondering?
directly across from the plane. Remember that all three of the p orbitals are in a right angle to one another. The third (leftover) p orbital will be perpendicular to the plane that the two p-orbitals form, just like the z axis is to the xy plane, depending on which two p-orbitals hybridise.
The unhybridized p-orbital in the case of BH3 and carbocations is empty.
However, there is another situation that occurs frequently where sp2 geometry is seen. A bond may form if the unhybridized p-orbitals of single electrons in two nearby atoms can overlap. The term "pi-bonding" refers to this phenomenon. (We'll talk about it in great detail later.)
Pi bonds—often just referred to as "double bonds"—need an unhybridized p orbital to form.
In the examples below, the carbon, oxygen, and nitrogen atoms—all of which have pi bonds (double bonds)—are sp2 hybridised. There is a 120 degree angle between the orbitals.
As we saw in NH3 and H2O in the case of sp3 hybridization, lone pairs can be in sp2-hybridized orbitals. Also observe that the nitrogen has one lone pair in a sp2 hybridised orbital in the top right molecule and one lone pair in a sp2 hybridised orbital in the middle molecule (formaldehyde).
Since a lone pair can be thought of as taking up more "room," the bond angles will be slightly less than 120° when one is present.
7. sp Hybridization
Let's look at the final scenario. What happens if just one p orbital combines with a s orbital?
This results in two hybrid " sp " orbitals that are 180 degrees apart, which is the maximum angle possible. This structure is referred to as "linear". Each hybrid sp orbital has a 50/50 s/p character composition.
The sp hybrid orbitals are at right angles to each of the two unhybridized p-orbitals.
Here are the orbitals in beryllium chloride (BeCl2), where the bond angle between the Cl and Be is 180 degrees. The two (unhybridized) p-orbitals will be along the y and z axes, respectively, if the Cl-Be-Cl bond is thought of as being along the x-axis.
When two pi bonds are present on a single atom, sp-hybridization is more frequently seen. The most well-known examples of "triple bonds" are carbon monoxide (CO), nitriles, and alkynes. In these situations, the oxygen and nitrogen atoms (in carbon monoxide) are also sp-hybridized in addition to the carbon atoms.
Also keep in mind that, as seen in nitriles and carbon monoxide, lone pairs can be in sp-hybridized orbitals.
Triple bonds are the most readily recognisable examples of sp-hybridization, though it is not limited to them. For instance, the central carbons in allene and ketene participate in two pi bonds and are sp-hybridized.
8. Summary – Hybrid Orbitals
OK. The highlights of this unintentionally lengthy post are as follows:
Electron pair opposition. The bond angles around CH4 would be limited to the p-orbital geometry (90°) in the absence of orbital hybridization. The formation of the sp3 orbitals, which result in a greater separation of the electron pairs and bond angles of 109° (i.e. at the apices of a tetrahedron), is energetically advantageous for the s and p orbitals to combine. This is also true for central atoms like NH3 and H2O that have electron pairs that do not form bonds.
Three sp2 hybrid orbitals with a trigonal planar orbital geometry are produced when only two p orbitals participate in the hybridization process. At right angles to the trigonal plane, the remaining (unhybridized) p orbital can either be empty (as in BH3) or singly occupied (as in molecules with pi bonds).
Two sp hybrid orbitals and a linear orbital geometry result from hybridization in which only one p orbital participates. Alkynes and nitriles are examples of organic compounds with three bonds that are available for pi-bonding, while BeCl2 has two empty p orbitals.
Keep in mind that there are always four orbitals surrounding the central atom. Hybridization only transforms orbitals; it neither creates nor destroys them.
We'll just give you a super easy trick to quickly ascertain the hybridization of a central atom in the following post.
Again, many thanks to Matt for contributing. Here, talk to Matt about arranging an online tutoring session.
Post a Comment