Formal charge is a bookkeeping term for the process of giving an atom a charge.
As a starting point, we count the number of valence electrons [Note 1] for the neutral atom. From there, we deduct the number of electrons it "owns" (i.e., electrons in lone pairs, or singly-occupied orbitals), as well as half of the electrons it shares (i.e., half the number of bonding electrons, which is equal to the number of bonds), to get the formal charge of the atom.
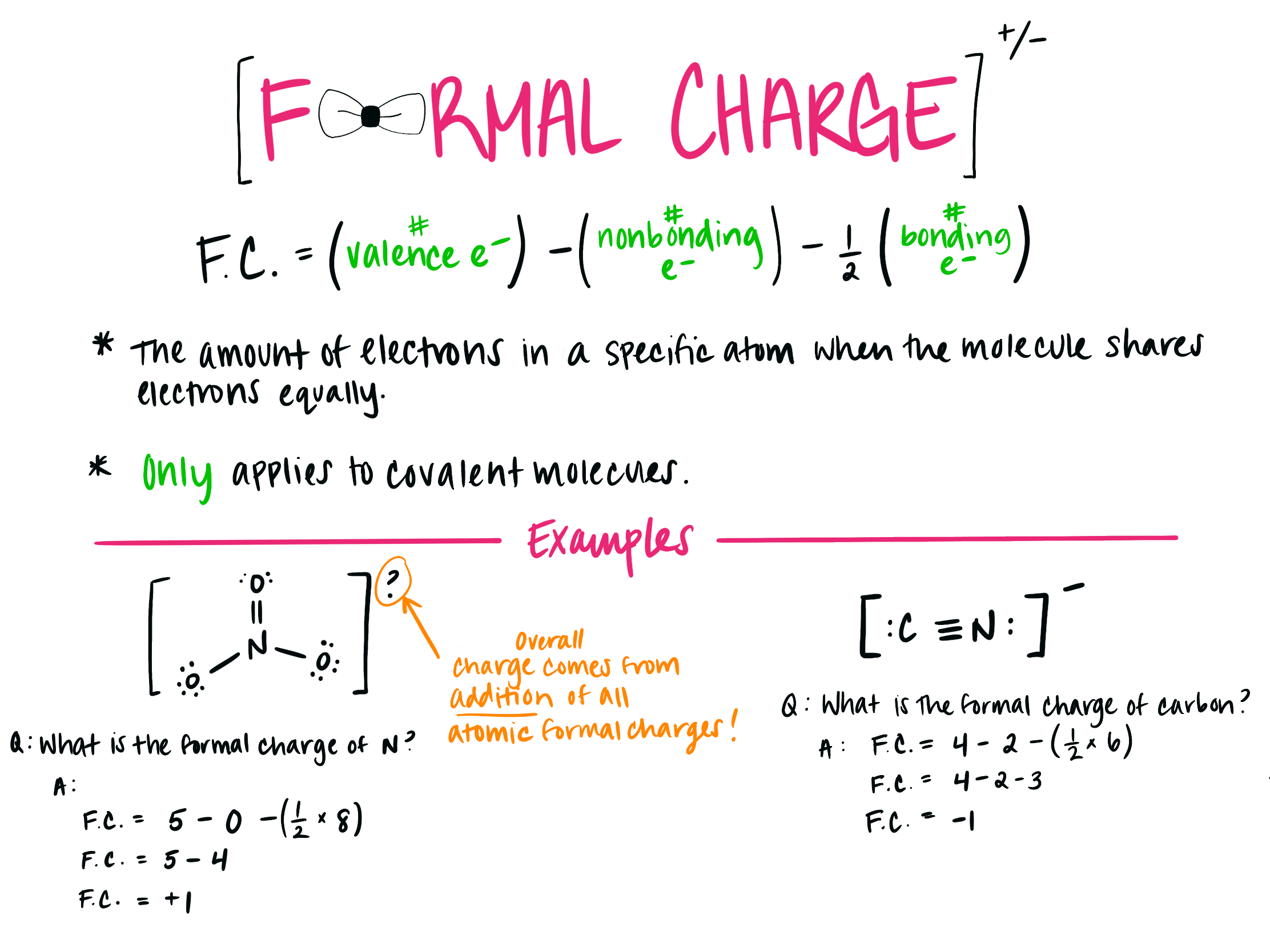
The formula for formal charge (FC) can be expressed most simply as follows:
FC = VE – NBE – B
where
The value of VE is determined by how many electrons are positioned around the neutral atom (3 for boron, 4 for carbon, 5 for nitrogen, 6 for oxygen, and 7 for fluorine).
NBE stands for non-bonded electrons, and the number of them around an atom is 2 for a lone pair, 1 for a single-occupied orbital, and 0 for an empty orbital.
B is the quantity of bonds surrounding the atom, which is equal to half of the bonding electrons.
Because it presumes that all bonding electrons are distributed equally, it is known as a "formal" charge. Dipoles, or variations in electronegativity, are not taken into account.
As a result, formal charge can be a poor indicator of reactivity and a poor indicator of where the electrons are actually located in a molecule. Below, we'll talk more about that.
2. Basic Illustrations Of First-Row Elements
Calculating formal charge is simple once you have all the lone pairs drawn out for you.
Let's take the quiz below and go over the first example.
The central atom of the hydronium ion (H3O) is oxygen, which has six valence electrons in the neutral atom.
The central atom has three bonds and two unpaired electrons.
H3O+ results from the formal charge on oxygen, which is [6 - 2 - 3 = +1].
Check to see if you can complete the examples below.
If everything went smoothly, you might try completing the formal charges for each example in this table.
Formal charge will take some getting used to, but after some time it will be assumed that you know how to calculate it and can spot structures with atoms that have a formal charge.
Let's tackle a few slightly more complex cases.
3. Formulations for Formal Charges When Not All Information Is Provided
Drawing a stick figure of a person without including their fingers doesn't necessarily depict that person as having a bad day at the table saw. We simply take it for granted that you could complete the fingers if absolutely necessary, but you're omitting it to save time.
Line drawings of chemicals resemble stick figures. They leave out a lot of information, but they still assume you are aware of some things.
We frequently skip drawing hydrogens with carbon. Still, you must be aware of their presence and add as many hydrogen atoms as required to complete an octet (or sextet, if the compound is a carbocation).
An electron on a carbon is always drawn in if it is a lone pair or unpaired electron.
One point. We can safely assume that a person only has 3 fingers if we draw a stick figure, draw the fingers, and then took the time to only draw in those 3. In order for you to know that the last two examples on that quiz were carbocations, we had to draw in the hydrogens; otherwise, you would have had to assume that they had a full octet!
Nitrogen and oxygen (as well as the halogens) are handled slightly differently.
Hydrogen bonds are always drawn in.
the single pairs that are frequently left out.
The octets of nitrogen and oxygen will always be full. Always. [Note 2: Two exceptions, okay]
Therefore, even if the lone pairs aren't drawn in, assume that there are enough to form an entire octet. Furthermore, if there are no bonds between these atoms and hydrogen, then there isn't any hydrogen at all.
Consider these instances:
See if you can combine these examples now!
(Take note that some of these are resonance forms rather than stable molecules, which you will come across throughout the course.)
4. A Few Conventional Charge Questions
To determine the formal charge of atoms in some rather unusual-looking molecules, we can use the same formal charge formula provided above along with the guidelines for implicit lone pairs and hydrogens.
Here are a few well-known formal charge issues.
The formal charge formula remains the same, despite the odd appearance of the structures.
Even some fairly unusual reactive intermediates that we'll encounter later in the semester can be charged using the formal charge formula.
Take care not to freak out. You can find the correct answer by simply counting the bonds and electrons.
5. Official Charges and S-shaped Arrows
To depict how electron pairs move during reactions and within resonance structures, we use curved arrows. (Read more in the post Curved Arrows For Reactions.)
Here is an illustration of a curved arrow that depicts the interaction of the proton (H+) and the hydroxide ion (HO(-)).
Two oxygen electrons move in the direction of the arrow to create a new O-H bond.
Curved arrows are helpful for tracking changes in formal charge as well. It is important to note that the formal charge at the oxygen at the curved arrow's initial tail increases from -1 to 0, while the formal charge at the H+ at the curved arrow's final tail decreases from +1 to 0.
The hydronium ion, H3O+, is created when acid and water are combined.
Here is a test. Try to depict the curved arrow that leads from the hydroxide ion to H3O+.
If you were successful, kudos to you!
However, I'm willing to wager that at least some of you drew the arrow pointing towards the positively charged oxygen.
What's incorrect about that?
On oxygen, there isn't an open orbital that will take the lone pair. The octet rule would be broken if you apply the logic of curved arrows, which would lead to a new O-O bond and 10 electrons on the oxygen.
Wait a second, you might say. I believed that oxygen had a positive charge. Where should it react if it doesn't react on oxygen?
The hydrogens are now! After all, Brnsted acid is H3O+. Right?
This is a great example of the difference between formal charge and electrostatic charge, also known as "partial charges" or "electron density," and why it is called "formal charge."
Assigning the "win" to one of the five pitchers in a baseball game is analogous to formal charge, which is ultimately just a book-keeping formality. [Note 3] It ignores the fact that the oxygen-hydrogen bond's electrons are unequally shared and have a sizable dipole.
The partial positive charges are all on hydrogen, despite the fact that we draw a "formal" charge on oxygen. has a partially negative electrostatic charge despite having a positive formal charge.
Because of this, bases like HO(-) react at the H rather than the oxygen.
Just to be clear:
Nitrogen and oxygen do not have empty orbitals when they are positively charged. Assume that nitrogen and oxygen both have complete octets! [Note 2]
Positive charges on carbon, however, do correspond to empty orbitals.
Halogens 6.
There are two main types of positive formal charges on halogens.
We frequently draw halonium ions as species with six valence electrons and an empty orbital, such as Cl+, Br+, and I+ (but never F+ because it's a ravenous beast).
Since these species are big and relatively polarizable, it's acceptable to think of them as having an empty orbital. They can disperse the positive charge across a sizable portion of their volume.
A full octet can be formed when these species accept a single pair of electrons from a Lewis base.
Due to their bonds to two atoms, Cl, Br, and I are also able to carry positive formal charges.
In these situations, it's critical to understand that the halogen bears a full octet, not an empty orbital. As a result, they won't readily accept a pair of electrons from Lewis bases; instead, the atom next to the halogen frequently does.
7. The Verdict
If you have completed the course and all of the quizzes, you should be well-prepared for all of the formal charge examples you encounter in the remaining portions of the course.
The formula FC = VE - NBE - B can be used to determine the formal charge.
Line drawings frequently omit C-H bonds and lone pairs. When determining formal charges, keep an eye out for these circumstances.
While positively charged nitrogen and oxygen are thought to have full octets, positively charged carbon has an empty orbital.
The hydronium ion H3O+ serves as an illustration of the dangers of relying solely on formal charge to comprehend reactivity. To truly understand an atom's reactivity, pay close attention to the variations in electronegativity among them and draw out the dipoles.
Post a Comment